ViiV Healthcare files submissions to the FDA and EMA for the first-ever dispersible formulation of dolutegravir (DTG) for children living with HIV
If approved, this new formulation of DTG will be the first integrase inhibitor available as a dispersible tablet for children living with HIV - closing the gap between treatment options available for adults and children
London, 13 December 2019 – ViiV Healthcare, the global specialist HIV company majority-owned by GSK, with Pfizer Inc. and Shionogi Limited as shareholders, today announced it has made regulatory submissions to both the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) seeking approval of the first-ever 5mg dispersible-tablet (DT) formulation of dolutegravir (DTG), as well as a simplified dosing regimen to optimise use of the existing DTG 50mg film-coated tablet (FCT) in paediatric HIV patients. The availability of age-appropriate formulations is essential in ensuring children around the world have access to optimal life-saving treatments.
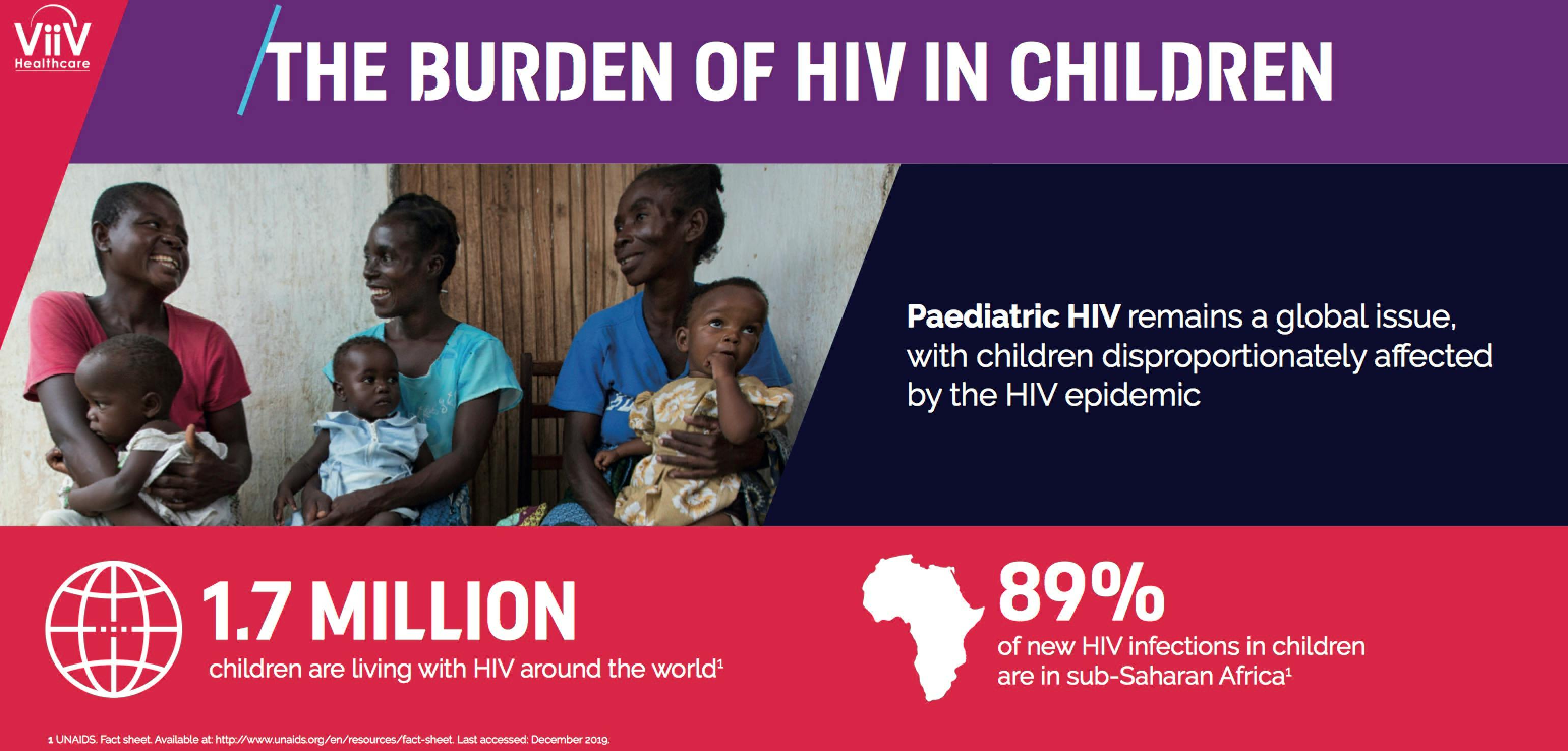
Paediatric HIV remains a global issue, with children disproportionately affected by the HIV epidemic. Latest statistics show there are 1.7 million children living with HIV1 , and the majority of AIDS-related deaths among children still occur during the first five years of life.2 Major obstacles persist for children, such as the availability of HIV testing, continued mother-to-child transmission, slow initiation of treatment and poor availability of optimised paediatric formulations of antiretrovirals.2,3

Deborah Waterhouse, CEO of ViiV Healthcare, said: “For parents living in resource poor countries, the ability to give medicine to children in a format that they can swallow and tolerate can mean the difference between life and death. By submitting these files to regulators for approval, we believe that we are on the cusp of delivering against our promise to develop dolutegravir in a tablet that can be dispersed simply in water. We will then aim to make this available, via partnerships, as quickly as possible to children living with HIV worldwide.”
These submissions to the EMA and FDA are based on data from the ongoing P10934 and ODYSSEY (PENTA20) studies.5 Data to support the submissions has been generated from ViiV Healthcare’s collaborations with the U.S. National Institutes of Health (NIH) and the International Maternal Paediatric Adolescent AIDS Clinical Trials Network (IMPAACT) for P1093 and the Paediatric European Network for Treatment of AIDS (Penta) for ODYSSEY.
Media contacts
For our corporate press office, email: Rachel Jaikaran
OR call +44 7823 523 755
For US-specific media enquiries,email: Audrey Abernathy
OR call +1 919 605 4521
Click the video to hear more from ViiV Healthcare’s Chief Medical Officer, Dr Harmony P. Garges.

Click the video to hear more from Chip Lyons, CEO, Elizabeth Glaser Paediatric AIDS Foundation President.
In order to support broad and more affordable access to optimised antiretroviral (ARV) formulations, ViiV Healthcare enables generic companies to manufacture and sell generic versions of paediatric DTG royalty-free in all least-developed, low-income, lower-middle-income and sub-Saharan African countries, as well as some upper-middle-income countries through its voluntary licensing policy. In addition to this, ViiV Healthcare has partnered with the Clinton Health Access Initiative (CHAI) and Unitaid since 2018 to expedite the development and introduction of optimised paediatric formulations of DTG by providing generic partners with financial and technical incentives to develop and manufacture it for resource-limited settings.

Notes to editors:
- P1093 (NCT03016533): investigates the safety, pharmacokinetic, tolerability and antiviral activity of DTG regimens in paediatric patients aged four weeks to 18 years and is being conducted by the IMPAACT network in the U.S., Brazil, Thailand, South Africa, Zimbabwe, and Malawi.
- ODYSSEY (PENTA20) (NCT02259127): is designed to understand how DTG as first- or second-line treatment compares to the current standards of care in paediatric patients aged four weeks to 18 years and is being conducted by the Penta network in Europe, South America, Thailand, Uganda, Zimbabwe, and South Africa. Originally designed to support the World Health Organization (WHO) guideline recommendations by WHO weight bands, this study will now provide data to support this submission. For more information, please visit the study website at: http://odysseytrial.org/
About dolutegravir
Dolutegravir (Tivicay) is an integrase strand transfer inhibitor (INSTI) for use in combination with other antiretroviral (ARV) agents for the treatment of HIV. Integrase inhibitors inhibit HIV integrase by binding to the integrase active site and blocking the strand transfer step of retroviral deoxyribonucleic acid (DNA) integration which is essential for the HIV replication cycle.
Tivicay is a registered trademark of the ViiV Healthcare group of companies. registration status of Tivicay worldwide. Click here for the latest registration status of Tivicay worldwide.
Important Safety Information (ISI) for dolutegravir 10, 25, and 50 mg tablets
The following Important Safety Information is based on the Summary of Product Characteristics (SmPC) for dolutegravir (Tivicay). Please consult the full SmPC for the full safety information.
Indications and Usage
Dolutegravir is a human immunodeficiency virus type 1 (HIV-1) integrase strand transfer inhibitor (INSTI) indicated in combination with:
- other ARV agents for the treatment of HIV-1 infection in adults and paediatric patients weighing at least 30 kg
- rilpivirine as a complete regimen for the treatment of HIV-1 infection in adults to replace the current ARV regimen in those who are virologically suppressed (HIV-1 RNA <50 copies per mL) on a stable ARV regimen for ≥6 months with no history of treatment failure or known substitutions associated with resistance to either ARV agent
Important Safety Information
CONTRAINDICATIONS
- Do not use dolutegravir in patients with previous hypersensitivity reaction to dolutegravir
- Do not use dolutegravir in patients receiving dofetilide
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions:
- Hypersensitivity reactions have been reported with dolutegravir and were characterized by rash, constitutional findings, and sometimes organ dysfunction, including liver injury
- Discontinue dolutegravir immediately if signs or symptoms of hypersensitivity reaction develop, as a delay in stopping treatment may result in a life-threatening reaction. Clinical status, including liver aminotransferases, should be monitored and appropriate therapy initiated
Hepatotoxicity:
- Hepatic adverse events have been reported, including cases of hepatic toxicity (elevated serum liver biochemistries, hepatitis, and acute liver failure) in patients receiving a dolutegravir containing regimen without pre-existing hepatic disease or other identifiable risk factors
- Patients with underlying hepatitis B or C or marked elevations in transaminases prior to treatment may be at increased risk for worsening or development of transaminase elevations with use of dolutegravir. In some cases, the elevations in transaminases were consistent with immune reconstitution syndrome or hepatitis B reactivation, particularly in the setting where anti-hepatitis therapy was withdrawn
- Monitoring for hepatotoxicity is recommended
Embryofetal Toxicity:
- Alternative treatments to dolutegravir should be considered at the time of conception through the first trimester of pregnancy due to the risk of neural tube defects
- Perform pregnancy testing before use of dolutegravir and counsel that consistent use of effective contraception is recommended while using dolutegravir in adolescents and adults of childbearing potential
Risk of Adverse Reactions or Loss of Virologic Response Due to Drug Interactions with concomitant use of dolutegravir and other drugs may result in known or potentially significant drug interactions (see Contraindications or Drug Interactions).
Immune Reconstitution Syndrome, including the occurrence of autoimmune disorders with variable time to onset, has been reported with the use of dolutegravir.
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥2%, Grades 2-4) in treatment-naïve adults receiving dolutegravir in a combination regimen were insomnia (3%), headache (2%), and fatigue (2%).
DRUG INTERACTIONS
- Coadministration of dolutegravir with drugs that induce or inhibit UGT1A1 and/or CYP3A may affect plasma concentrations
- Administer dolutegravir 2 hours before or 6 hours after taking antacids, polyvalent cation-containing products or laxatives, sucralfate, oral supplements containing iron or calcium, or buffered medications. Alternatively, oral calcium and iron supplements (including multivitamins containing calcium or iron) can be taken with dolutegravir if co-administered with a meal
- Consult the full Prescribing Information for dolutegravir for more information on potentially significant drug interactions, including clinical comments
USE IN SPECIFIC POPULATIONS
- Pregnancy: There are insufficient human data on the use of dolutegravir during pregnancy to definitively assess a drug-associated risk for birth defects and miscarriage. An Antiretroviral Pregnancy Registry has been established. If planning a pregnancy or if pregnancy is confirmed while taking dolutegravir during the first trimester, assess the risks and benefits of continuing dolutegravir versus switching to another ARV regimen. For individuals actively trying to become pregnant, initiation of dolutegravir is not recommended unless there is no suitable alternative
- Lactation: Breastfeeding is not recommended due to the potential for HIV-1 transmission, developing viral resistance in HIV-positive infants, and adverse reactions in a breastfed infant
- Females and Males of Reproductive Potential: Perform pregnancy testing before initiation of dolutegravir. Advise adolescents and adults of childbearing potential to consistently use effective contraception while taking dolutegravir
Please see accompanying full Product Information for more details.
Full U.S. prescribing information including is available at: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Informatio n/Tivicay/pdf/TIVICAY-PI-PIL.PDF
For the EU Summary of Product Characteristics, please visit: https://www.ema.europa.eu/en/documents/product-information/tivicay-epar-productinformation_en.pdf
About The International Maternal Paediatric Adolescent AIDS Clinical Trials (IMPAACT) Network
The International Maternal Paediatric Adolescent AIDS Clinical Trials (IMPAACT) Network is a global collaboration of investigators, institutions, community representatives and other partners organised for the purpose of evaluating interventions to treat and prevent HIV infection and its consequences in infants, children, adolescents and pregnant/postpartum women through the conduct of high-quality clinical trials.
The IMPAACT Network's research agenda includes evaluation of: new and existing anti-HIV drugs and formulations; novel approaches for addressing tuberculosis in HIV-infected or at-risk populations; biomedical/behavioural interventions to prevent mother-to-child HIV transmission; immunogenicity, safety and efficacy of high priority vaccines; potential for HIV cure through therapeutic interventions; and methods to prevent and manage complications and comorbidities of HIV infection and its treatment.
For more information on IMPAACT visit: https://impaactnetwork.org/
About Penta
Penta is a global independent scientific network dedicated to research in child health. Starting out from its work in HIV, today Penta’s portfolio includes investigation into other infectious diseases affecting children, as well as infrastructure development initiatives and training programmes. Since 2012, Penta has sponsored 35 clinical trials, with more than 50,000 women and children enrolled into its studies. The work of Penta is supported by a network of 110 clinical sites in 31 countries across the world whose expertise are leveraged to transform the prevention and treatment of infection in children.
About ViiV Healthcare
ViiV Healthcare is a global specialist HIV company established in November 2009 by GlaxoSmithKline (LSE: GSK) and Pfizer (NYSE: PFE) dedicated to delivering advances in treatment and care for people living with HIV and for people who are at risk of becoming infected with HIV. Shionogi joined in October 2012. The company’s aim is to take a deeper and broader interest in HIV/AIDS than any company has done before and take a new approach to deliver effective and innovative medicines for HIV treatment and prevention, as well as support communities affected by HIV.
For more information on the company, its management, portfolio, pipeline and commitment, please visit www.viivhealthcare.com
About GSK
GSK is a science-led global healthcare company with a special purpose: to help people do more, feel better, live longer. For further information please visit www.gsk.com.
Cautionary statement regarding forward-looking statements
GSK cautions investors that any forward-looking statements or projections made by GSK, including those made in this announcement, are subject to risks and uncertainties that may cause actual results to differ materially from those projected. Such factors include, but are not limited to, those described under Item 3.D 'Principal risks and uncertainties' in the company's Annual Report on Form 20-F for 2018.
ViiV Healthcare Media enquiries: |
Isabelle Scali |
+44 (0) 7557 290420 |
GSK Global Media enquiries: |
Simon Moore Director, Vaccines & Global Health Corporate Media |
+44 (0) 20 8047 5502 |
|
Kristen Neese |
+1 804 217 8147 |
| Analyst/Investor enquiries: | Sarah Elton-Farr |
+44 (0) 20 8047 5194 |
|
Danielle Smith |
+44 (0) 20 8047 0932 |
|
James Dodwell |
+44 (0) 20 8047 2406 |
|
Jeff McLaughlin |
+1 215 751 7002 |
References:
- UNAIDS. Global HIV & AIDS statistics – 2019 fact sheet. Available at: https://www.unaids.org/en/resources/fact-sheet Last accessed: November 2019.
- UNAIDS. Get on the fast-track. The life-cycle approach to HIV. Available at: https://www.unaids.org/sites/default/files/media_asset/Get-on-the-Fast-Track_en.pdf Last accessed: November 2019.
- UNAIDS. Children and HIV– 2016 Fact Sheet. Available at: https://www.unaids.org/sites/default/files/media_asset/FactSheet_Children_en.pdf Last accessed: November 2019
- Clinicaltrials.gov. (2019). PH3b, DTG Study in HIV-1 Subjects Completing IMPAACT Study P1093. Available at: https://clinicaltrials.gov/ct2/show/NCT03016533 Last accessed: November 2019.
- Clinicaltrials.gov. (2019). A Randomised Trial of Dolutegravir (DTG)-Based Antiretroviral Therapy vs. Standard of Care (SOC) in Children With HIV Infection Starting First-line or Switching to Second-line. Available at: https://clinicaltrials.gov/ct2/show/NCT02259127?term=PENTA20&draw=2&rank=1 Last accessed: November 2019.
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in the package leaflet. You can also report side effects directly via the GSK Reporting Tool link https://gsk.public.reportum.com/. By reporting side effects, you can help provide more information on the safety of this medicine.
If you are from outside the UK, you can report adverse events to GSK/ ViiV by selecting your region and market, here.