TRANSFORMATIVE PARTNERSHIP BETWEEN THE MEDICINES PATENT POOL AND ViiV HEALTHCARE ENABLES 24 MILLION PEOPLE IN LOW- AND MIDDLE-INCOME COUNTRIES TO ACCESS INNOVATIVE HIV TREATMENT
- 10 years on, access-oriented voluntary licensing agreements between ViiV Healthcare and the Medicines Patent Pool, and directly with Aurobindo Pharma, have enabled large scale access to dolutegravir, a WHO-recommended antiretroviral for treatment of people living with HIV. This partnership has also evolved to include preventative HIV medicine.
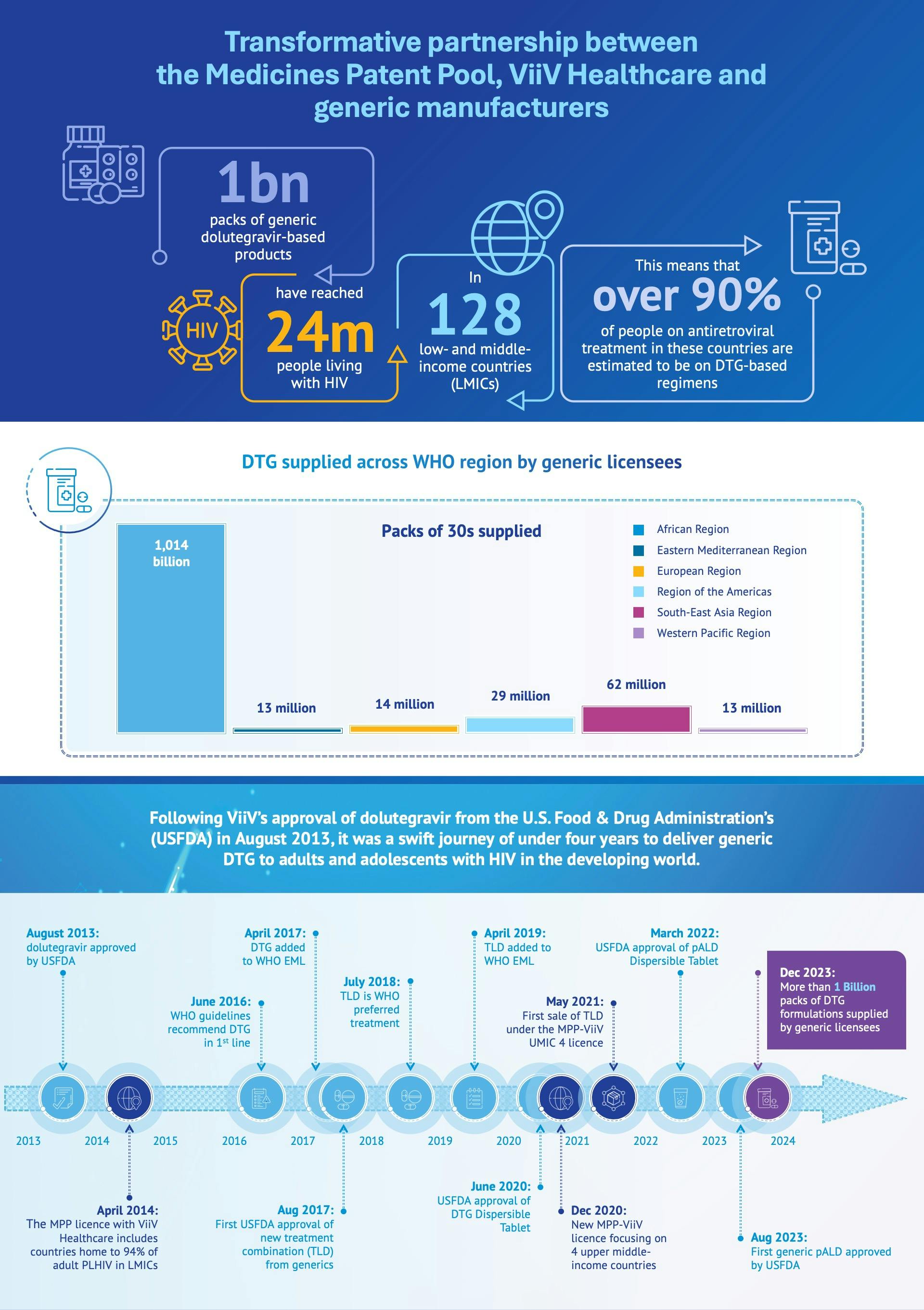
London, 30 April 2024 – ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, together with the Medicines Patent Pool (MPP), today announce a major milestone in their partnership. Ten years after the signing of ground-breaking licensing agreements between ViiV Healthcare and the Medicines Patent Pool (MPP), as well as the direct agreements with Aurobindo Pharma and ViiV Healthcare, more than 1 billion packs of generic dolutegravir (DTG)- based medicines have reached 24 million people living with HIV in 128 low- and middle-income countries (LMICs), transforming the HIV landscape in those areas of the world. This means that as of 2023, over 90% of people on antiretroviral treatment in these countries are estimated to be on DTG-based regimens.
Thanks to this decade-long partnership, WHO-recommended treatment can be developed, produced, and when approved, supplied by 15 generic manufacturers to all low-income, least developed, lower-middle-income and sub-Saharan African countries, as well as some upper middle-income countries, addressing the needs of regions with the highest HIV burdens.
Charles Gore, Executive Director, Medicines Patent Pool said: "As we reflect on a decade of collaboration with ViiV Healthcare, we are proud to see that public health-oriented voluntary licensing has had tangible impact in the lives of the people we serve, enabling access to affordable versions of innovative HIV treatments in LMICs. We commend ViiV Healthcare for their huge commitment, allowing our partnership to demonstrate that voluntary licensing can serve as a sustainable strategy to enhance affordable production and distribution for more equitable access to essential medicines in underserved territories.”
Deborah Waterhouse, CEO, ViiV Healthcare said: "The scale and impact of our 10-year collaboration with the Medicines Patent Pool and with Aurobindo Pharma are testament to the power of partnership in advancing global health. We are proud to have played a pivotal role in enabling 24 million people living with HIV access affordable dolutegravir-based treatments, and we look forward to our continued collaboration with MPP and generic partners as we broaden the focus to include innovative preventative HIV medicines."
Philippe Duneton, Executive Director, Unitaid said: "When Unitaid established the Medicines Patent Pool in 2010, many doubted whether voluntary licensing was possible. Yet, originator companies came to the negotiating table, proving that this partnership model works. Thanks to the MPP and ViiV Healthcare's licensing agreements, millions of people with HIV in LMICs now have access to affordable treatments that come at a much lower cost. Unitaid is proud to support this decade-long partnership, and we look forward to expanding our efforts to make innovative health solutions accessible to all.”
Nombeko Mpongo, Community Liaison Administrator at the Desmond Tutu HIV Centre in South Africa, said: “What this partnership has done to help expand access to innovative HIV medicines has profoundly impacted my life and the lives of countless others in South Africa. The widespread availability of TLD (tenofovir, lamivudine, dolutegravir – TDF/3TC/DTG) underscores the remarkable progress we’ve made in HIV treatment accessibility and affordability.”
Partnership accelerates access to innovative HIV medicines for paediatrics.
As well as enabling widespread access to TLD (a WHO-preferred treatment for adults and adolescents), this 10-year partnership played a foundational role in the development and availability of age-appropriate DTG-based treatment options for children and infants, addressing a key gap as children are disproportionately affected by HIV – with paediatric treatment coverage still lagging behind adults.1 As a result of MPP and ViiV’s licensing agreement, as well as a novel public-private partnership between ViiV, the Clinton Health Access Initiative (CHAI) and Unitaid providing technology transfer and regulatory support, generic dispersible tablet formulations of DTG have now been supplied to 95 LMICs for children weighing at least 3kg.
Most recently, in 2023, three generic dispersible formulations of paediatric ALD (abacavir, lamivudine, dolutegravir – ABC/3TC/DTG) received approval for infants from three months of age and weighing at least 6kg. This was further to an additional partnership programme which ViiV and CHAI drove with generic partners, in parallel with ViiV’s own paediatric development programme for ABC/3TC/DTG (the first dispersible single-tablet regimen containing dolutegravir). Introduction of this newer combination in LMICs will further support delivery of HIV medication to young children and address unmet needs by offering a reduced pill burden.
Arvind Kanda, Head of ARV/API South Africa and Sub-Saharan Africa at Viatris said: “We are proud of our partnership with the Medicines Patent Pool and ViiV Healthcare, a collaboration that has enabled us to make a significant impact in the fight against HIV. Our journey began with pioneering the TLD regimen for adults with HIV, where we were the first MPP licensee to receive tentative USFDA approval under the U.S. President's Emergency Plan for AIDS Relief (PEPFAR). But our commitment doesn’t end there. We are equally dedicated to advancing access to the most innovative HIV treatments for children. Through our partnerships to accelerate development and production of paediatric formulations we are addressing the unique needs of children living with HIV. We remain unwavering in our mission: empowering people worldwide to live healthier at every stage of life.”
Sister Tresa Palakudy, Manager, Nyumbani Children’s Home, Kenya, said: “Caring for children living with HIV at Nyumbani, I’m truly grateful that Kenya has had access to paediatric dolutegravir since 2021 through this transformational partnership. I want to offer my heartfelt thanks to ViiV Healthcare, the Medicines Patent Pool, Unitaid, and all the international health partners for making this innovation accessible to our children living with HIV in LMICs.”
Partnership continues with new voluntary licence to include first-of-its-kind preventative medicine.
Looking ahead, ViiV Healthcare and MPP are building on this decade-long partnership through furthering access to innovative HIV prevention. The signing of a new licence agreement in 2022 marked a significant step forward in driving access to cabotegravir long-acting for PrEP, and ViiV is supporting all generic sublicensees with technical support and know-how to expedite product development and enable access to this novel prevention tool for people in LMICs.
Notes to the editors
The decade-long collaboration between MPP, ViiV Healthcare and generic partners has been a beacon of progress in the fight against HIV, enabling access to innovative WHO-recommended treatments including:
- ALD - ABC/3TC/DTG (600/300/50 mg) – abacavir/lamivudine/dolutegravir: Fixed-dose combination antiretroviral regimen recommended by WHO as an option that may be considered as first-line treatment of HIV in adults and adolescents (as well as other usages – see here).
- ALD (paediatric) - ABC/3TC/DTG (60/30/5 mg) dispersible – abacavir/lamivudine/dolutegravir: Fixed-dose combination antiretroviral regimen recommended by WHO as the preferred first-line treatment of HIV in children above 25kg (as well as other usages – see here).
- DTG adult (50 mg) – dolutegravir adults: Antiretroviral recommended by WHO as part of the preferred first-line treatment of HIV in adults, adolescents, complemented with TDF + 3TC (or FTC), in adults and adolescents (as well as other usages – see here).
- DTG paediatric (50 mg) – dolutegravir paediatrics: Antiretroviral recommended by WHO as part of the preferred first-line treatment of HIV in children above 20kg, complemented with ABC + 3TC (see here).
- DTG paediatric (10 mg) scored dispersible – dolutegravir paediatrics: Antiretroviral recommended by WHO as part of the preferred first-line treatment of HIV in children from four weeks and 3 kg, complemented with ABC + 3TC (see here).
- TAF/3TC/DTG (25/300/50 mg) – tenofovir alafenamide/lamivudine/dolutegravir: Fixed-dose combination antiretroviral regimen recommended by WHO as an option that may be considered as first-line treatment of HIV in adults and adolescents with established osteoporosis and/or impaired kidney function (as well as other usages – see here).
- TAF/FTC/DTG (25/200/50 mg) – tenofovir alafenamide/emtricitabine/dolutegravir: Fixed-dose combination antiretroviral regimen recommended by WHO as an option that may be considered as first-line treatment of HIV in adults and adolescents with established osteoporosis and/or impaired kidney function (as well as other usages – see here).
- TLD - TDF/3TC/DTG (300/300/50 mg) – tenofovir disoproxil fumarate/lamivudine/dolutegravir: Fixed-dose combination antiretroviral regimen recommended by WHO as the preferred first-line treatment of HIV in adults and adolescents (see here).
In 2023, The Global Fund established a new benchmark price for TLD in low- and middle-income countries (LMICs) of under US$45 per person per year, a 40% decrease from the $75 dollar price of 2017, when a pricing agreement was announced following regulatory approval of the first generic formulations of TLD. This cost-effective strategy has empowered governments in resource-constrained settings to broaden their HIV care services.
About Tivicay
Tivicay contains dolutegravir, an integrase strand transfer inhibitor for use in combination with other antiretroviral agents for the treatment of HIV. Integrase inhibitors inhibit HIV integrase by binding to the integrase active site and blocking the strand transfer step of retroviral deoxyribonucleic acid (DNA) integration which is essential for the HIV replication cycle.
Please see full US Prescribing Information for Tivicay.
About Triumeq
Triumeq is a fixed-dose combination containing the integrase strand transfer inhibitor (INSTI) dolutegravir and the nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) abacavir and lamivudine.
Two essential steps in the HIV life cycle are replication – when the virus turns its RNA copy into DNA – and integration – the moment when viral DNA becomes part of the host cell’s DNA. These processes require two enzymes called reverse transcriptase and integrase. NRTIs and integrase inhibitors interfere with the action of the two enzymes to prevent the virus from replicating and further infecting cells.
Please see full US Prescribing Information for Triumeq.
About cabotegravir extended-release injectable suspension
Cabotegravir LA for HIV prevention is the first and only long-acting injectable PrEP option proven superior to daily oral FTC/TDF in reducing HIV acquisition.
Cabotegravir LA for PrEP is an integrase strand transfer inhibitor (INSTI). INSTIs, like cabotegravir extended-release injectable suspension, inhibit HIV replication by preventing the viral DNA from integrating into the genetic material of human immune cells (T-cells). This step is essential in the HIV replication cycle and is also responsible for establishing chronic disease.
Cabotegravir LA for PrEP is provided as an injection administered six times per year and is initiated with a single 600 mg (3-ml) injection given one month apart for two consecutive months. After the second initiation injection, the recommended continuation injection dose is a single 600 mg (3-ml) injection given every two months. Cabotegravir oral tablets may be administered for approximately one month before initiating the first injection to assess the tolerability of the medicine.
Please see full US Prescribing Information for Apretude.
Trademarks are owned by or licensed to the ViiV Healthcare group of companies.
About the Medicines Patent Pool
The Medicines Patent Pool (MPP) is a United Nations-backed public health organisation working to increase access to, and facilitate the development of, life-saving medicines for low- and middle-income countries. Through its innovative business model, MPP partners with civil society, governments, international organisations, industry, patient groups, and other stakeholders, to prioritise and license needed medicines and pool intellectual property to encourage generic manufacture and the development of new formulations. To date, MPP has signed agreements with 22 patent holders for 13 HIV antiretrovirals, one HIV technology platform, three hepatitis C direct-acting antivirals, a tuberculosis treatment, a cancer treatment, four long-acting technologies, a post-partum haemorrhage medicine, three oral antiviral treatments for COVID-19 and 16 COVID-19 technologies. MPP was founded by Unitaid, which continues to be MPP’s main funder. MPP’s work on access to essential medicines is also funded by the French Ministry for Europe and Foreign Affairs, the German Agency for International Cooperation, the Japanese Government, and the Swiss Agency for Development and Cooperation. More information at https://medicinespatentpool.org and follow us on X, LinkedIn and YouTube.
About ViiV Healthcare
ViiV Healthcare is a global specialist HIV company established in November 2009 by GSK (LSE: GSK) and Pfizer (NYSE: PFE) dedicated to delivering advances in treatment and care for people living with HIV and for people who are at risk of acquiring HIV. Shionogi became a ViiV shareholder in October 2012. The company’s aims are to take a deeper and broader interest in HIV and AIDS than any company has done before and take a new approach to deliver effective and innovative medicines for HIV treatment and prevention, as well as support communities affected by HIV.
For more information on the company, its management, portfolio, pipeline, and commitment, please visit viivhealthcare.com.
About GSK
GSK is a global biopharma company with a purpose to unite science, technology, and talent to get ahead of disease together. Find out more at gsk.com.
| MPP enquiries: | |||
| Sophie Thievenaz press@medicinespatentpool.org |
+41 79 870 85 52 | (Geneva) | |
| ViiV Healthcare enquiries: | |||
| Media enquiries: | Rachel Jaikaran |
+44 (0) 78 2352 3755 | (London) |
| Audrey Abernathy | +1 919 605 4521 | (North Carolina) | |
| Melinda Stubbee | +1 919 491 0831 | (North Carolina) | |
| GSK enquiries: | |||
| Media: | Tim Foley | +44 (0) 20 8047 5502 | (London) |
| Sarah Clements | +44 (0) 20 8047 5502 |
(London) | |
| Kathleen Quinn | +1 202 603 5003 | (Washington DC) | |
| Lyndsay Meyer | +1 202 302 4595 | (Washington DC) | |
| Alison Hunt | +1 540 742 3391 | (Washington DC) | |
| Investor Relations: | Nick Stone | +44 (0) 7717 618834 | (London) |
| James Dodwell | +44 (0) 20 8047 2406 | (London) | |
| Mick Readey | +44 (0) 7990 339653 | (London) | |
| Josh Williams | +44 (0) 7385 415719 | (London) | |
| Camilla Campbell | +44 (0) 7803 050238 | (London) | |
| Steph Mountifield | +44 (0) 7796 707505 | (London) | |
| Jeff McLaughlin | +1 215 751 7002 | (Philadelphia) | |
| Frannie DeFranco | +1 215 751 4855 | (Philadelphia) | |
Cautionary statement regarding forward-looking statements
GSK cautions investors that any forward-looking statements or projections made by GSK, including those made in this announcement, are subject to risks and uncertainties that may cause actual results to differ materially from those projected. Such factors include, but are not limited to, those described under Item 3.D “Risk factors” in the company's Annual Report on Form 20-F for 2023.
Registered in England & Wales:
GSK plc ViiV Healthcare Limited
No. 3888792 No. 06876960
Registered Office:
GSK plc ViiV Healthcare Limited
980 Great West Road GSK Medicines Research Centre
Brentford, Middlesex Gunnels Wood Road, Stevenage
United Kingdom United Kingdom
TW8 9GS SG1 2NY
References
- Adopted by United Nations Member States in June 2021, alongside ambitious targets for primary prevention and supporting enablers, the 95–95–95 HIV testing, treatment and viral suppression targets aim to close gaps in HIV treatment coverage and outcomes in all sub-populations, age groups and geographic settings. https://www.unaids.org/sites/default/files/media_asset/progress-towards-95-95-95_en.pdf (accessed April 2024)
- Global HIV & AIDS statistics — Fact sheet | UNAIDS
Media contacts
For our corporate press office, email: Rachel Jaikaran
OR call +44 7823 523 755
For US-specific media enquiries, email: Audrey Abernathy
OR call +1 919 605 4521
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in the package leaflet. You can also report side effects directly via the GSK Reporting Tool link https://gsk.public.reportum.com/. By reporting side effects, you can help provide more information on the safety of this medicine.
If you are from outside the UK, you can report adverse events to GSK/ ViiV by selecting your region and market, here.